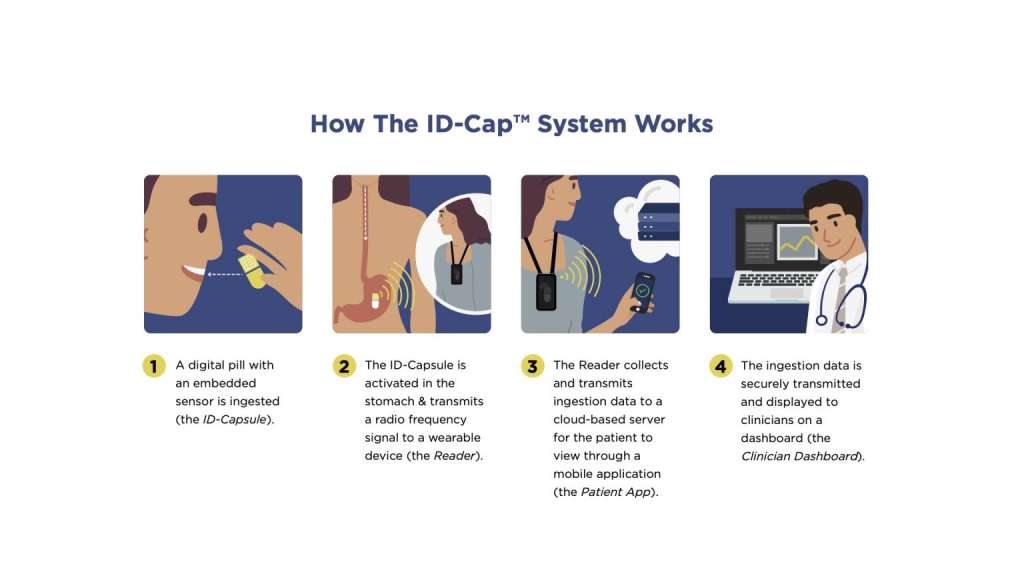
etectRx announces record-breaking use of the ID-Cap™ System, utilized in University of Colorado’s HIV treatment study. The Quantification of Tenofovir Alafenamide Adherence and Exposure in Adults living with HIV (QUANTI-TAF) study evaluated drug concentrations in dried blood spots (DBS), in tandem with medication adherence measured by the ID-Cap™ System. The ID-Cap™ System’s remote patient monitoring (RPM) capabilities successfully enabled the QUANTI-TAF study to produce one of the largest and longest digital pill studies in HIV treatment history.
A seamless, end-to-end solution, the ID-Cap™ is the only ingestible sensor solution which allowed the study’s researchers to monitor, track, trend, report, and provide real-time, accurate data through etectRx’s core technology, eBurst. When nonadherence was detected by the ingestible sensor technology, University of Colorado researchers were able to intervene in a timely fashion to send real-time dosing reminders to patients’ smartphone apps.
QUANTI-TAF began in August 2020, and the last follow-up concluded in May 2023. A total of 84 participants were enrolled in the study for up to 4 months each, resulting in 8,910 expected ingestions over 9,049 days monitored, and a cumulative adherence rate of 95%. The QUANTI-TAF study showed consistently high acceptability of the ID-Cap™ System over time, as participants used it for up to approximately 112 days (4 months).
Data from University of Colorado’s QUANTI-TAF study demonstrates significant milestones for the ID-Cap™ System with a reputation for demonstrating strong results and providing data to accurately track patients’ adherence to their oral medications. “The ID-Cap™ System was central to our QUANTI-TAF study; we used the digital pill data to establish adherence benchmarks for our drug concentrations, which was a key outcome of our study,” said Peter Anderson, PharmD- Antiviral Pharmacology, Professor at University of Colorado Anshutz Medical Campus.
etectRx gained FDA clearance for its innovative ID-Cap™ System technology in December 2019. Since FDA clearance, the technology has generated strong, accurate results across multiple indications, particularly with patient populations with traditionally poor levels of medication adherence, including those with substance abuse disorders.
About etectRx™
etectRx is a digital health company. The FDA-cleared ID-Cap™ System uniquely addresses the issue with adherence to (oral) medication. The accurate, flexible, and simple digital pill system allows researchers, pharmacists, physicians, and pharmaceutical companies to rethink approaches to medication adherence, innovate with confidence, and accelerate patient outcomes. To learn more, visit https://etectrx.com/.

